Kids With Cystic Fibrosis Baby With Vest for Cystic Fibrosis
Introduction
Newborn screening (NBS) programs were start established nigh 60 years ago in the United States after the seminal discovery that phenylalanine could be detected from a dried blood spot, ultimately leading to early diagnosis of phenylketonuria and avoidance of the severe neurocognitive complications feature of this inherited metabolic disorder (1). Other screening programs emerged, generally adhering to basic principles outlined in a report commissioned by the World Health Organization (two), and the number of diseases tested has grown in the last several decades. While at that place is variability between programs, some states and countries screen for every bit many as 50 treatable metabolic conditions, endocrinopathies, hemoglobinopathies, and genetic diseases, like cystic fibrosis (CF).
Occurring in roughly 1 in iii,000 live births in the United states, based on epidemiological and neonatal screening information, CF is the well-nigh common, life-shortening inherited disease of Caucasians (3). CF is acquired by defective CF transmembrane conductance regulator (CFTR), a cAMP-regulated anion transporter expressed on the surface of various epithelia. Functionally linked to the epithelial sodium aqueduct and other apical channels, CFTR abnormalities lead to reduced epithelial chloride conductance and sodium absorption, resulting in dehydration of the periciliary fluid layer and mucus on the airway surface that impairs mucociliary clearance (4, 5). Innate defenses are besides compromised by altered bicarbonate secretion in the CF airway (half dozen, 7). Together, these changes lead to progressive airway obstruction, allowing bacterial infection to go established and provoking a persistent neutrophilic inflammatory response that results in the gradual destruction of the airways and ultimately respiratory failure.
Earlier implementation of NBS for CF, children were typically diagnosed after developing symptoms consistent with fatty malabsorption, often leading to nutritional failure. Some were identified presently later on birth when they presented with meconium ileus, which occurs in roughly 15% of children with CF (8, 9). Recurrent respiratory infections, often misdiagnosed as asthma or bronchiolitis, would occur during infancy, but are more common in older children. Indeed, children with milder, pancreatic sufficient phenotypes are often recognized later as their respiratory symptoms become more prevalent.
Adoption of NBS in many nations has led to earlier diagnosis and treatment, and the life expectancy of a child born with CF in many parts of the world has steadily improved (10, 11). In improver, newer, small molecule therapeutics have begun to dramatically alter the illness (12, thirteen). In this commodity, we will review NBS for CF and describe existing and emerging therapies that accept impacted the progressive respiratory decline of people with CF and how they may avoid lung disease and other complications even before they brainstorm.
NBS For CF
Early on attempts to screen neonates for CF over xl years ago relied on measuring albumin content in dried meconium (14), which had a loftier faux-positive rate. Even so, it was discovered that young infants with CF had elevated, circulating levels of pancreatic enzymes and proenzymes, even children with pancreatic sufficient forms of the affliction. In particular, trypsinogen, a pancreatic enzyme forerunner released from the inflamed exocrine pancreas caused by inspissated secretions and destruction of acinar cells, can be detected in the blood of neonates with CF. Indeed, over twoscore years ago, Crossley and colleagues showed that the serum immunoreactive trypsinogen (IRT) could exist measured in claret spots dried on the Guthrie cards (15), and the ability to measure this analyte was paramount for development of a broad, population-based newborn programme.
In the United States, methods for NBS differ between states and countries, only all invariably use some form of the IRT measurements every bit function of the screening procedure. It is important to note that IRT concentrations can exist elevated in the absence of CF, particularly in neonates who are premature, have depression Apgar scores, or feel perinatal stress. It was recognized that a single-tier approach had a lower sensitivity, then in the United states, near states have adopted 2-tier protocols that involve serial IRT measurements repeated 1 to 2 weeks autonomously if the initial value is elevated, or IRT followed by genetic testing for specific CFTR mutations if abnormal. Some states have adopted a third-tier, using a protocol that involves repeated measurements of IRT levels, with DNA analysis performed if both concentrations are above the designated threshold (xvi, 17). Uniquely, California, a state that has a racially diverse population, has incorporated CFTR sequencing in their screen to identify CF in neonates with loftier IRT concentrations and merely ane mutation in their genetic panel (18).
The threshold defining an elevated IRT level varies between states. While some states will apply an absolute concentration to prompt farther testing, others use percentile cutoffs that improve specificity. Serial IRT approaches without genetic analysis has benefits, allowing for identification of individuals with less mutual CFTR mutations in certain populations (19), merely delaying fourth dimension to a positive screen because the 2d specimen is obtained later. Genetic panels used in NBS are variable, and the number of CFTR mutations analyzed may differ from country-to-state. Genetic testing has the advantage of identifying people who are heterozygous for CFTR mutations, but as well more probable to identify patients with mutations but normal or equivocal sweat chloride levels, referred to as CFTR-related metabolic syndrome or, in Europe, CF screen-positive, inconclusive diagnosis.
Adoption and Evolution of CF NBS
In the Usa, NBS for CF was slowly accepted, given the relative absenteeism of data showing benefits of early diagnosis. Indeed, fifty-fifty the Cystic Fibrosis Foundation was hesitant to make a recommendation regarding NBS, stating the benefits of presymptomatic and early handling were controversial (20). Nevertheless, screening programs were established in North America. In 1982, Colorado became the showtime state to implement NBS for CF, followed before long thereafter past Wisconsin. Initially developed equally part of a decade-long randomized controlled trial (21), NBS was added to the Wisconsin country-wide program in 1994 (22). A workshop held by the Centers for Disease Control and Prevention and Cystic Fibrosis Foundation in 2003 evaluated diagnostic testing and conclusion-making and provided recommendations for all-time practices for screening for CF (23). At the time of its publication, only 8 states had implemented an NBS programme, merely within vii years, all l states and Washington, DC, had screening programs for CF, with Texas beingness the last country to implement screening.
Like to the The states experience, there is considerable variability in screening programs among nations. Certainly, affliction prevalence plays an influential part in the need for screening and early diagnosis (24, 25). Worldwide, Australia and New Zealand were pioneers, establishing NBS programs in 1981 (26). During the past two decades, the number of programs has rapidly increased in Europe (25, 27), with more twenty European countries performing NBS at some level. Indeed, alternative screening approaches have been adopted in some countries. For instance, a iv-tier screening algorithm was created in the Netherlands that involves measurement of IRT and pancreatitis-associated poly peptide levels from the same dried blood spot (28), CFTR mutation panel, and, if indicated, extended genomic analysis. The Dutch feel highlights the complexity of such programs, and a reminder NBS is exactly that, a screening tool and not a diagnostic test.
Often overlooked, successful NBS depends on the accuracy of diagnostic testing. The diagnosis of CF is based on elevated sweat chloride concentrations (29). Any clinical concerns for CF, regardless of the screening result, are an indication for sweat chloride measurement. While other approaches are available, the but reliable, validated diagnostic examination for measuring sweat chloride concentration is the quantitative pilocarpine iontophoresis exam, performed according to Clinical and Laboratory Standards Institute guidelines (xxx).
Benefits of NBS For CF
NBS, regardless of disease, is successful but if early on identification is feasible using simple, cost-effective means and tin can pb to improved clinical outcomes. For decades, the diagnosis of CF required clinical suspicion. Before screening, the median age of diagnosis was vi months in the United States, and nearly 70% of affected children were identified by their first birthday (31). Malnutrition occurs early in life (32), and pulmonary involvement can begin early in infancy, despite the child appearing asymptomatic (33, 34). With widespread adoption of NBS in the United states of america, the age at diagnosis has shifted to <1 month, often before the child is symptomatic.
There was early bear witness from the netherlands almost five decades ago that screening in the neonatal period was associated with a survival do good (35). While CF was added relatively late to US programs, there was growing evidence that delayed diagnosis would take serious implications for affected people. Considering of depression mortality rates in children, it is difficult to establish survival differences betwixt screened and unscreened children with CF. Using information from the United states of america Cystic Fibrosis Foundation Patient Registry of more than 27,000 patients, children identified by screening inside a month of age and treated early had ameliorate survival compared to counterparts diagnosed symptomatically (36), supported by several subsequent studies (37–39).
The best evidence conspicuously showing benefits of NBS is its event on early nutrition and growth. Before screening, children with CF were often diagnosed afterward becoming severely malnourished with vitamin deficiencies. These children oft failed to achieve their growth potential and had evidence of dumb evolution of cognitive office, probable related to malnutrition. Investigators in Wisconsin unsurprisingly plant that the diagnosis of CF was confirmed by a positive sweat exam at a much younger historic period in a screened cohort as compared to controls. Moreover, children with CF identified past screening had significantly meliorate height, weight, and head circumference percentiles, and these differences persisted throughout infancy and early childhood, especially the children who had pancreatic insufficiency and homozygous for the Phe508del mutation (40). Recently, a multicenter, longitudinal, observational cohort study examined the nutritional health of 231 American children with CF identified by NBS over a iii-yr period and found significant improvement in nutritional condition, with normalization of weight in the first year of life (41).
Malnourished children with CF have increased risk of chronic lung affliction. A large report of 931 children with CF examining the effect of early diet on the development of lung illness highlighted the importance of earlier intervention. Children with better nutritional indices at 3 years of historic period had college lung function measures at the age of half dozen years (42). Thus, early diagnosis in order to optimize the nutritional condition by starting enzymes at diagnosis and adding nutritional supplements as indicated tin lead to improved pulmonary health.
In that location have been several studies that show children diagnosed with CF following NBS accept fewer complications than those who were symptomatic at the time of diagnosis. Australian investigators compared the outcomes of children with CF identified early on from NBS or diagnosed tardily. Unscreened patients had reduced height, lower pulmonary function measures, and increased rates of infection and colonization with Pseudomonas aeruginosa (43). Respiratory benefits persisted into adolescence (44, 45). Others have similarly shown benefits in the US and UK populations, with reduced P. aeruginosa infections, reduced handling burden, and fewer hospitalizations (46–48). These findings emphasize non only the importance of early diagnosis and detection, but too the demand for continued improvement of screening protocols with genetic advances.
In improver to the clinical benefits from early identification, screening programs for CF take economical benefits, with several studies revealing its price-effectiveness (49, 50). Additionally, investigators have reported that the incidence or CFTR allele distribution decreased following implementation of NBS, although these observations demand to be confirmed in larger studies (51, 52).
Implications of NBS In The ERA of Modulator Therapies
Direction of CF has been directed at the downstream consequences of CFTR dysfunction, incorporating antibiotics, anti-inflammatory agents, inhaled mucolytics, and airway clearance techniques. Pancreatic enzyme replacement therapy and vitamin supplements treat pancreatic insufficiency and prevent nutritional deficits. These treatments have led to longer lives, even before widespread implementation of NBS. Nonetheless, the emergence of novel small molecule therapeutics that target the basic defects has raised promise that CF lung disease tin can be prevented before it starts. These newer CFTR potentiators and correctors have mutation-specific effects that can restore CFTR function. These agents are revolutionizing care and have reduced respiratory symptoms, exacerbation frequency, and slowed progression of lung illness in people with CF (12, 13).
When ivacaftor was approved by the US Nutrient and Drug Administration (FDA) 8 years ago, preceding clinical trials showed dramatic improvements in sweat chloride values along with improvements in weight gain, pulmonary exacerbation rates, and lung function measures in patients with G551D mutations, a class 3 CFTR gating defect (12, 53). Subsequent studies revealed improved lung mucociliary clearance that was sustained 3 months following treatment, which correlated with forced expiratory volume in 1 due south (FEV1) (54). More recently, several studies then evaluated the effectiveness of ivacaftor in younger children (55–57). A stage 3, multicenter trial examined the ivacaftor pharmacokinetics in young children and its effect on sweat chloride concentrations, growth parameters, and markers of exocrine pancreatic part. Growth measures for historic period were normal throughout the study, and pancreatic function biomarkers also improved, suggesting that ivacaftor preserved exocrine pancreatic role (56). Other studies have shown benign nutritional effects in preschool children (55, 58). These findings were surprising. In CF, the exocrine pancreas is involved earlier birth, with obstacle of small-scale ducts and acini seen as early equally the second trimester (59). Affliction progresses after birth, with pancreatic inflammation, fibrosis, and fatty infiltration, in one case thought to occur in early infancy (60).
In secondary analyses of GOAL (G551D Observational Study), lung clearance indices were significantly improved in treated children inside 1 calendar month of starting treatment and were maintained 6 months after beginning therapy (61). In fact, ivacaftor is now canonical for utilise in children with sure mutations every bit young as 4 months of age.
Since the development of ivacaftor (Kalydeco (R)), three other therapies have been canonical for use in the United States (62). Lumacaftor–ivacaftor (Orkambi®) was initially approved for utilize in 2015 for those with homozygous Phe508del mutations and is now available for employ down to 2 years of age. Tezacaftor–ivacaftor (Symdeko®) was approved in 2018 and may now exist used for those 6 years or older who have homozygous Phe508del mutations or Phe508del and a second specific mutation. Most recently, highly effective triple-drug therapy, elexacaftor–tezacaftor–ivacaftor (Trikafta® or Kaftrio®), was approved past the FDA and more recently the European Commission for utilise in CF patients 12 years or older who have one or two Phe508del mutations, which accounts for 90% of all affected individuals (13, 63). This treatment has shown the most hope in altering the clinical trajectory of those with CF. Trials showed marked reductions of sweat chloride and improved pct predicted FEVi values (xiii).
Modulator therapy is increasingly used in younger children and even infants, raising the prospect that CF could be prevented before it begins (55, 56). Yet, to be successful, primary prevention requires early on diagnosis and treatment. NBS is an essential part of the success of early diagnosis and with the advent of modulator therapy use in younger and younger children, critically important.
Given these improvements, although purely speculative, primary prevention may exist doable. Using genetically modified ferrets that harbor CFTR G551D mutations, investigators showed the potential benefits of CFTR modulators (64). Like other creature models for CF, the newborn ferret is decumbent to meconium ileus, with lxxx% experiencing severe abdominal obstruction that leads to early death. Notwithstanding, when pregnant jills were treated with ivacaftor (VX-770) late in pregnancy, kits homozygous for the G551D mutation had markedly reduced incidence of neonatal bowel obstruction.
Postnatally, ivacaftor was administered to the kits, and they maintained pancreatic sufficiency and grew equally well every bit wild-type littermates. In chemical compound heterozygous (G551D/KO) ferrets, nearly remained pancreatic insufficient, merely many maintained normal growth. Similarly, ivacaftor treatment protected the airways from bacterial infection and inflammation (Figure i). In one case treatment was discontinued; nevertheless, the benefits disappeared, and CF kits developed characteristic pancreatic and pulmonary pathology. These findings suggest the importance of early and sustained modulator treatment in maintaining CFTR function (65), and these agents are not a cure.
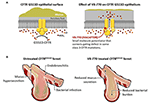
Effigy 1. Early on treatment with VX-770 prevents pathological changes in a cystic fibrosis animal model. (A) Result of the CFTR potentiator VX-770 (ivacaftor) on ion channel gating of the CFTR G551D mutation. The G551D mutation abolishes ATP-dependent gating, which results in reduced channel open probability, only treatment with VX-770 alters activity of the mutant CFTR, leading to greater chloride ion and bicarbonate ion secretion, reduced sodium ion absorption, and hydration of epithelial surfaces. (B) Effect of prenatal and postnatal handling with VX-770 (ivacaftor) on the airways of immature ferrets with G551D mutation. Mucus aggregating, bacterial infection, and endobronchitis develop early in untreated airways of young kits with G551D mutations, only treated animals avoided bronchial infection and inflammation until the drug was discontinued. Similar effects were seen in other affected organs, including the pancreas, intestines, and genitourinary tract. Modified from Ferkol (65).
While fertility was not assessed, the vas deferens and epididymis appeared pathologically normal in male kits homozygous for the G551D mutation, in contrast to compound heterozygous (G551D/KO) ferrets. Thus, 1 could speculate obstructive azoospermia or congenital bilateral absence of the vas deferens could exist prevented in certain patients. The pathogenesis of the male genitourinary defects begins in utero, likely related to accumulation of obstructing, thickened secretions that leads to degeneration of the vas deferens. Indeed, male fetuses with CF, betwixt 12- and 18-week gestation, accept a normal vas deferens, demonstrating that the defect occurs later in embryonic development (66).
It would exist premature to consider clinical trials testing the efficacy of ivacaftor in preventing CF in neonates who take G551D. First, there would be few eligible subjects. Few people with CF are homozygous for course 3 CFTR defects (53). Moreover, treating a fetus by treating an unaffected pregnant mother would pose ethical bug; significant women and their unborn children are ofttimes excluded from pharmaceutical trials. These therapies are not without risk, including liver dysfunction and cataract evolution, and would likely prohibit use in an unaffected adult female.
That said, nosotros may presently take testify of whether principal prevention of CF is viable. In contrast to their male counterparts who take obstructive azoospermia, women with CF are generally fertile, and with improvements in intendance, a growing proportion are having children. Many women with CF are being treated with the newer, highly constructive triple combination therapy, elexacaftor–tezacaftor–ivacaftor (13, 63). To maintain the female parent's pulmonary and nutritional health, they frequently continue treatment throughout pregnancy at many centers.
While partners of pregnant women with CF typically undergo prenatal testing for CFTR mutations, occasionally they are missed, and children are born with CF. If their unborn child has CF and Phe508del mutation(s), he/she would indirectly be treated with elexacaftor–tezacaftor–ivacaftor in utero, as these small molecules can cross the placental barrier, thus leading to several interesting questions. Would combination therapy in this child forestall progressive airway disease, maintain pancreatic sufficiency, or preserve male fertility, paralleling what was described in the ferret model (65)? How would 1 appraise the latter in young infants who typically do non have respiratory symptoms (67), and what would we utilise to demonstrate a treatment effect in the lung (67)? For primary prevention strategies to succeed, sensitive outcome measures are needed to find the primeval changes in lung disease in infants and young children.
Furthermore, would it be unethical to withdraw a drug that prevented illness one time the baby is born, despite lack of regulatory approval for young infants? If and so, in the absence of clinical trials, how would we decide optimal dosing in this population?
Finally, would CFTR correction interfere with NBS of children born to women with CF, resulting in a false-negative screen? Could CFTR correction attenuate pancreatic injury and issue in a negative IRT level? Nosotros may demand to rethink our screening and diagnostic approach for such children.
While there are many significant gaps in available diagnostics and treatments between countries (68), nosotros take entered a new era in CF, full of promise and possibilities. To achieve this potential, effective screening and diagnostic testing must be in place. Prenatal and neonatal screening programs hateful that infants can be diagnosed and interventions begun before they are symptomatic. In some countries, CFTR genotyping is frequently performed early on in life, and mutation- or grade-specific CFTR modulators accept already changed the lives of older infants and children. What was in one case unimaginable could become reality—primary prevention of CF might be achievable.
Author Contributions
Ac composed the first draft and did not receive an honorarium or grant to write the manuscript. Both authors listed on the manuscript have reviewed, approved the content of the submission, and have full responsibility for the information provided.
Funding
The authors were supported by National Institutes of Health (NIH) awards U54HL096458 (TF) and R21AI46999 (TF) and the Cystic Fibrosis Foundation.
Disclaimer
The views expressed practice not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention past merchandise names, commercial practices, or organizations imply endorsement by the U.S regime.
Disharmonize of Interest
The authors declare that the enquiry was conducted in the absence of whatsoever commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
cAMP, circadian adenosine monophosphate; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator; FEV1, forced expiratory book in one 2d; IRT, immunoreactive trypsinogen; NBS, newborn screening.
References
ane. Guthrie R, Susi A. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics. (1963) 32:338–43.
PubMed Abstruse | Google Scholar
2. Wilson JMG, Jungner G. Principles and practice of screening for disease. Who Chron. (1968) 22:473.
Google Scholar
iii. Michelson P, Faro A, Ferkol T. Pulmonary affliction in cystic fibrosis. In: Wilmott R, Bush A, Ratjen F, et al., editors. Kendig'southward Disorders of the Respiratory Tract in Children. 9th Edn. Philadelphia, PA: Elsevier Health Sciences (2018). p. 777–87.
Google Scholar
4. Verkman As, Vocal Y, Thiagarajah JR. Office of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol Prison cell Physiol. (2003) 284:C2–xv. doi: ten.1152/ajpcell.00417.2002
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Shah VS, Meyerholz DK, Tang XX, Reznikov Fifty, Abou Alaiwa Yard, Ernst SE, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. (2016) 351:503–7. doi: 10.1126/science.aad5589
PubMed Abstract | CrossRef Total Text | Google Scholar
7. Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. (2012) 487:109–13. doi: 10.1038/nature11130
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Dupuis A, Keenan Grand, Ooi CY, Dorfman R, Sontag MK, Naehrlich 50, et al. Prevalence of meconium ileus marks the severity of mutations of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene. Genet Med. (2016) 18:333–twoscore. doi: x.1038/gim.2015.79
PubMed Abstruse | CrossRef Total Text | Google Scholar
9. Rosenstein BJ, Langbaum TS. Incidence of meconium abnormalities in newborn infants with cystic fibrosis. Am J Dis Child. (1980) 134:72–three. doi: 10.1001/archpedi.1980.02130130054016
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Dankert-Roelse JE, te Meerman GJ. Long term prognosis of patients with cystic fibrosis in relation to early on detection by neonatal screening and handling in a cystic fibrosis centre. Thorax. (1995) 50:712–8. doi: 10.1136/thx.l.vii.712
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2018. Annual Data Report. Bethesda, MD (2019).
PubMed Abstract | Google Scholar
12. Ramsey BW, Davies J, McElvaney NG, Tullis East, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. North Engl J Med. (2011) 365:1663–72. doi: x.1056/NEJMoa1105185
PubMed Abstract | CrossRef Total Text | Google Scholar
13. Middleton PG, Mall MA, Drevinek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor-Tezacaftor-Ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. (2019) 381:1809–19. doi: 10.1056/NEJMoa1908639
PubMed Abstruse | CrossRef Full Text | Google Scholar
16. Currier RJ, Sciortino S, Liu R, Bishop T, Alikhani Koupaei R, Feuchtbaum Fifty. Genomic sequencing in cystic fibrosis newborn screening: what works best, two-tier predefined CFTR mutation panels or second-tier CFTR panel followed by third-tier sequencing? Genet Med. (2017) 19:1159–63. doi: 10.1038/gim.2017.32
PubMed Abstract | CrossRef Full Text | Google Scholar
17. Sontag MK, Lee R, Wright D, Freedenberg D, Sagel SD. Improving the sensitivity and positive predictive value in a cystic fibrosis newborn screening program using a repeat immunoreactive trypsinogen and genetic analysis. J Pediatr. (2016) 175:150–8.e1. doi: 10.1016/j.jpeds.2016.03.046
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Kharrazi M, Yang J, Bishop T, Lessing Southward, Immature S, Graham S, et al. Newborn screening for cystic fibrosis in California. Pediatrics. (2015) 136:1062–72. doi: 10.1542/peds.2015-0811
PubMed Abstract | CrossRef Total Text | Google Scholar
19. Castellani C, Massie J, Sontag M, Southern KW. Newborn screening for cystic fibrosis. Lancet Respir Med. (2016) 4:653–61. doi: 10.1016/S2213-2600(16)00053-ix
CrossRef Total Text | Google Scholar
twenty. Ad hoc Committee Task Force on Neonatal Screening, Cystic Fibrosis Foundation, Taussig LM, Boat TF, Dayton D, Fost Northward, Hammond K, et al. Neonatal screening for cystic fibrosis: position paper. Pediatrics. (1983) 72:741–5.
PubMed Abstract | Google Scholar
21. Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns WT, et al. Nutritional benefits of neonatal screening for cystic fibrosis. wisconsin cystic fibrosis neonatal screening study group. N Engl J Med. (1997) 337:963–9. doi: 10.1056/NEJM199710023371403
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Rock MJ, Hoffman G, Laessig RH, Kopish GJ, Litsheim TJ, Farrell PM. Newborn screening for cystic fibrosis in Wisconsin: nine-twelvemonth experience with routine trypsinogen/Dna testing. J Pediatr. (2005) 147:S73–7. doi: 10.1016/j.jpeds.2005.08.004
PubMed Abstruse | CrossRef Total Text | Google Scholar
23. Grosse SD, Boyle CA, Botkin JR, Comeau AM, Kharrazi G, Rosenfeld M, et al. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. MMWR Recomm Rep. (2004) 53:i–36.
PubMed Abstract | Google Scholar
24. Castellani C, Duff AJA, Bell SC, Heijerman HGM, Munck A, Ratjen F, et al. ECFS all-time exercise guidelines: the 2018 revision. J Cyst Fibros. (2018) 17:153–78. doi: ten.1016/j.jcf.2018.02.006
PubMed Abstract | CrossRef Full Text | Google Scholar
26. Massie J, Clements B, Australian Paediatric Respiratory G. Diagnosis of cystic fibrosis afterwards newborn screening: the Australasian feel–20 years and five million babies later: a consensus argument from the Australasian paediatric respiratory group. Pediatr Pulmonol. (2005) 39:440–half-dozen. doi: 10.1002/ppul.20191
PubMed Abstruse | CrossRef Total Text | Google Scholar
27. Barben J, Castellani C, Dankert-Roelse J, Gartner South, Kashirskaya Northward, Linnane B, et al. The expansion and performance of national newborn screening programmes for cystic fibrosis in Europe. J Cyst Fibros. (2017) 16:207–thirteen. doi: ten.1016/j.jcf.2016.12.012
PubMed Abstruse | CrossRef Full Text | Google Scholar
28. Dankert-Roelse JE, Bouva MJ, Jakobs BS, Janssens HM, de Winter-de Groot KM, Schonbeck Y, et al. Newborn blood spot screening for cystic fibrosis with a 4-footstep screening strategy in holland. J Cyst Fibros. (2019) 18:54–63. doi: x.1016/j.jcf.2018.07.008
PubMed Abstract | CrossRef Full Text | Google Scholar
29. LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ Jr. Cystic Fibrosis Foundation. Diagnostic sweat testing: the cystic fibrosis foundation guidelines. J Pediatr. (2007) 151:85–ix. doi: ten.1016/j.jpeds.2007.03.002
PubMed Abstruse | CrossRef Full Text | Google Scholar
30. LeGrys V, Gibson L, Hammond R, Kraft K, Rosenstein B. Sweat Testing: Sample Collection and Quantitative Assay; Canonical Guideline. Wayne, PA: US National Commission for Clinical Laboratory Standards (2009).
Google Scholar
31. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry 2011. Annual Data Report. Bethesda, MD (2012).
PubMed Abstract | Google Scholar
32. Pedersen MG, Hojte C, Olesen HV, Pressler T, Skov Grand. Late diagnosis and poor nutrition in cystic fibrosis diagnosed before implementation of newborn screening. Acta Paediatr. (2019) 108:2241–v. doi: ten.1111/apa.14908
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Sly PD, Brennan S, Gangell C, de Klerk North, Murray C, Mott L, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Intendance Med. (2009) 180:146–52. doi: 10.1164/rccm.200901-0069OC
PubMed Abstract | CrossRef Full Text | Google Scholar
34. Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, et al. Progression of early structural lung disease in immature children with cystic fibrosis assessed using CT. Thorax. (2012) 67:509–16. doi: x.1136/thoraxjnl-2011-200912
PubMed Abstract | CrossRef Total Text | Google Scholar
35. Dankert-Roelse JE, te Meerman GJ, Martijn A, ten Kate LP, Knol K. Survival and clinical issue in patients with cystic fibrosis, with or without neonatal screening. J Pediatr. (1989) 114:362–7. doi: 10.1016/S0022-3476(89)80552-9
CrossRef Full Text | Google Scholar
36. Lai HJ, Cheng Y, Farrell PM. The survival advantage of patients with cystic fibrosis diagnosed through neonatal screening: evidence from the U.s. cystic fibrosis foundation registry data. J Pediatr. (2005) 147:S57–63. doi: x.1016/j.jpeds.2005.08.014
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Grosse SD, Rosenfeld M, Devine OJ, Lai HJ, Farrell PM. Potential bear upon of newborn screening for cystic fibrosis on child survival: a systematic review and analysis. J Pediatr. (2006) 149:362–six. doi: 10.1016/j.jpeds.2006.04.059
PubMed Abstruse | CrossRef Full Text | Google Scholar
38. Dijk FN, McKay Yard, Barzi F, Gaskin KJ, Fitzgerald DA. Improved survival in cystic fibrosis patients diagnosed by newborn screening compared to a historical cohort from the same middle. Curvation Dis Child. (2011) 96:1118–23. doi: x.1136/archdischild-2011-300449
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Tridello K, Castellani C, Meneghelli I, Tamanini A, Assael BM. Early on diagnosis from newborn screening maximises survival in astringent cystic fibrosis. ERJ Open Res. (2018) 4:00109–2017. doi: 10.1183/23120541.00109-2017
PubMed Abstract | CrossRef Full Text | Google Scholar
twoscore. Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng Fifty, Lai HC, et al. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Wisconsin cystic fibrosis neonatal screening written report group. Pediatrics. (2001) 107:1–thirteen. doi: ten.1542/peds.107.1.1
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Leung DH, Heltshe SL, Borowitz D, Gelfond D, Kloster M, Heubi JE, et al. Furnishings of diagnosis by newborn screening for cystic fibrosis on weight and length in the commencement year of life. JAMA Pediatr. (2017) 171:546–54. doi: x.1001/jamapediatrics.2017.0206
PubMed Abstract | CrossRef Full Text | Google Scholar
42. Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, et al. Growth and nutritional indexes in early on life predict pulmonary function in cystic fibrosis. J Pediatr. (2003) 142:624–30. doi: 10.1067/mpd.2003.152
PubMed Abstruse | CrossRef Total Text | Google Scholar
43. Coffey MJ, Whitaker Five, Gentin N, Junek R, Shalhoub C, Nightingale S, et al. Differences in outcomes between early on and late diagnosis of cystic fibrosis in the newborn screening era. J Pediatr. (2017) 181:137–45.e1. doi: x.1016/j.jpeds.2016.x.045
PubMed Abstract | CrossRef Full Text | Google Scholar
44. McKay KO, Waters DL, Gaskin KJ. The influence of newborn screening for cystic fibrosis on pulmonary outcomes in new South Wales. J Pediatr. (2005) 147:S47–50. doi: 10.1016/j.jpeds.2005.08.013
PubMed Abstract | CrossRef Total Text | Google Scholar
45. Martin B, Schechter MS, Jaffe A, Cooper P, Bong SC, Ranganathan S. Comparison of the Us and Australian cystic fibrosis registries: the impact of newborn screening. Pediatrics. (2012) 129:e348–55. doi: ten.1542/peds.2011-0567
PubMed Abstract | CrossRef Full Text | Google Scholar
46. Accurso FJ, Sontag MK, Wagener JS. Complications associated with symptomatic diagnosis in infants with cystic fibrosis. J Pediatr. (2005) 147:S37–41. doi: 10.1016/j.jpeds.2005.08.034
PubMed Abstract | CrossRef Total Text | Google Scholar
47. Sims EJ, McCormick J, Mehta G, Mehta A, Committee UCDS. Newborn screening for cystic fibrosis is associated with reduced handling intensity. J Pediatr. (2005) 147:306–xi. doi: 10.1016/j.jpeds.2005.05.034
PubMed Abstract | CrossRef Full Text | Google Scholar
48. Sims EJ, McCormick J, Mehta G, Mehta A, Steering Commission of the UKCFD. Neonatal screening for cystic fibrosis is benign even in the context of modern handling. J Pediatr. (2005) 147:S42–6. doi: x.1016/j.jpeds.2005.08.002
PubMed Abstract | CrossRef Full Text | Google Scholar
49. Nshimyumukiza L, Bois A, Daigneault P, Lands Fifty, Laberge AM, Fournier D, et al. Cost effectiveness of newborn screening for cystic fibrosis: a simulation study. J Cyst Fibros. (2014) xiii:267–74. doi: x.1016/j.jcf.2013.ten.012
PubMed Abstract | CrossRef Full Text | Google Scholar
50. van der Ploeg CP, van den Akker-van Marle ME, Vernooij-van Langen AM, Elvers LH, Gille JJ, Verkerk PH, et al. Cost-effectiveness of newborn screening for cystic fibrosis determined with real-life information. J Cyst Fibros. (2015) 14:194–202. doi: x.1016/j.jcf.2014.08.007
PubMed Abstract | CrossRef Total Text | Google Scholar
52. Castellani C, Picci L, Tamanini A, Girardi P, Rizzotti P, Assael BM. Association between carrier screening and incidence of cystic fibrosis. JAMA. (2009) 302:2573–ix. doi: x.1001/jama.2009.1758
PubMed Abstract | CrossRef Full Text | Google Scholar
53. Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Outcome of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. (2010) 363:1991–2003. doi: 10.1056/NEJMoa0909825
PubMed Abstruse | CrossRef Full Text | Google Scholar
54. Donaldson SH, Laube BL, Corcoran TE, Bhambhvani P, Zeman One thousand, Ceppe A, et al. Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR. JCI Insight. (2018) three:e122695. doi: 10.1172/jci.insight.122695
PubMed Abstract | CrossRef Full Text | Google Scholar
55. Rosenfeld M, Cunningham Due south, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. An open-characterization extension study of ivacaftor in children with CF and a CFTR gating mutation initiating treatment at age 2-5years (KLIMB). J Cyst Fibros. (2019) 18:838–43. doi: 10.1016/j.jcf.2019.03.009
PubMed Abstract | CrossRef Total Text | Google Scholar
56. Rosenfeld G, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, et al. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (Inflow): a stage 3 unmarried-arm study. Lancet Respir Med. (2018) 6:545–53. doi: x.1016/S2213-2600(18)30202-ix
PubMed Abstract | CrossRef Full Text | Google Scholar
57. Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, et al. Efficacy and safe of ivacaftor in patients aged half dozen to eleven years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. (2013) 187:1219–25. doi: x.1164/rccm.201301-0153OC
PubMed Abstract | CrossRef Full Text | Google Scholar
58. Davies JC, Cunningham Due south, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2-5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. (2016) four:107–15. doi: 10.1016/S2213-2600(15)00545-seven
PubMed Abstract | CrossRef Full Text | Google Scholar
60. Imrie JR, Fagan DG, Sturgess JM. Quantitative-Evaluation of the development of the exocrine pancreas in cystic-fibrosis and control infants. Am J Pathol. (1979) 95:697–707.
PubMed Abstruse | Google Scholar
61. Ratjen F, Klingel M, Black P, Powers MR, Grasemann H, Solomon M, et al. Changes in lung clearance index in preschool-aged patients with cystic fibrosis treated with ivacaftor (GOAL): a clinical trial. Am J Respir Crit Care Med. (2018) 198:526–8. doi: 10.1164/rccm.201802-0243LE
PubMed Abstract | CrossRef Full Text | Google Scholar
62. Egan ME. Cystic fibrosis transmembrane conductance receptor modulator therapy in cystic fibrosis, an update. Curr Opin Pediatrics. (2020) 32:384–8. doi: 10.1097/MOP.0000000000000892
PubMed Abstract | CrossRef Full Text | Google Scholar
63. Heijerman HGM, McKone EF, Downey DG, Van Braeckel East, Rowe SM, Tullis East, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, stage 3 trial. Lancet. (2019) 394:1940–8. doi: ten.1016/S0140-6736(19)32597-8
PubMed Abstract | CrossRef Total Text | Google Scholar
64. Sun 10, Yi Y, Yan Z, Rosen BH, Liang B, Winter MC, et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci Transl Med. (2019) 11:eau7531. doi: 10.1126/scitranslmed.aau7531
PubMed Abstract | CrossRef Total Text | Google Scholar
67. Pittman JE, Cutting G, Davis SD, Ferkol T, Boucher R. Cystic fibrosis: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. (2014) 11(Suppl. 3):S161–8. doi: 10.1513/AnnalsATS.201312-444LD
PubMed Abstract | CrossRef Full Text | Google Scholar
68. Bell SC, Mall MA, Gutierrez H, Macek One thousand, Madge S, Davies JC, et al. The future of cystic fibrosis intendance: a global perspective. Lancet Respir Med. (2020) 8:65–124. doi: x.1016/S2213-2600(19)30337-half-dozen
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fped.2020.608821/full
0 Response to "Kids With Cystic Fibrosis Baby With Vest for Cystic Fibrosis"
Post a Comment